
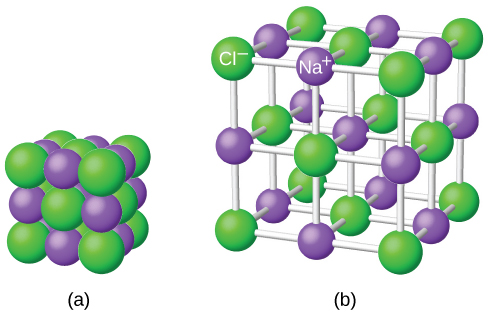
By chance we might just as well have centered the diagram around a chloride ion - that, of course, would be touched by 6 sodium ions. The sodium ion in the center is being touched by 6 chloride ions. Only those ions joined by lines are actually touching each other.

We normally draw an "exploded" version which looks like this: This diagram is easy enough to draw with a computer, but extremely difficult to draw convincingly by hand. If you look at the diagram carefully, you will see that the sodium ions and chloride ions alternate with each other in each of the three dimensions. A small representative bit of a sodium chloride lattice looks like this: That is different from, say, a water molecule which always contains exactly 2 hydrogen atoms and one oxygen atom - never more and never less. There could be billions of sodium ions and chloride ions packed together, or trillions, or whatever - it simply depends how big the crystal is. It means that you can't state exactly how many ions there are. You should be clear that giant in this context does not just mean very large. So sodium chloride (and any other ionic compound) is described as having a giant ionic structure. Compounds like this consist of a giant (endlessly repeating) lattice of ions. Sodium chloride is taken as a typical ionic compound. The structure of a typical ionic solid - sodium chloride Often these are nonstoichiometric or complex stoichiometries with ordered vacancies (Cr 7S 8, Fe 7S 8).\) For example, some compounds with the NiAs structure are: MS, MSe, MTe (M=Ti, V, Fe, Co, Ni). This structure is mainly adopted by covalent and polar covalent MX compounds, typically with "soft" X anions (S, Se, P, As.) and low-valent transition metal cations. The NiAs structure cannot be adopted by ionic compounds because of the eclipsing cations, because the cation-cation repulsions would be internally destabilizing for an ionic compound. The layer stacking sequence for NiAs is shown below: Unlike the NaCl structure, where the anion and cation sites are interchangeable, NiAs has unique anion and cation sites. In terms of layer stacking, the NiAs structure is AcBcAcBc., where the A and B sites (the hcp lattice) are occupied by the As atoms, and the c sites, which are eclipsed along the layer stacking axis, are occupied by Ni. The anions are also coordinated to six cations, but they occupy trigonal prismatic sites. The cations are in octahedral coordination, so each cation is coordinated to six anions. The cations are shown in gray while the anions are light blue in the figure at the right. This is the structure adopted by NiAs and many other transition metal sulfides, phosphides, and arsenides. One octahedron of six As atoms surrounding a Ni atom is shown in the center of the figure. The Ni 6As trigonal prisms are shaded gray. What if we start with a hexagonal-close packed lattice rather than a face-centered cubic lattice? The NaCl structure can be described a face-centered cubic lattice with all of the octahedral holes filled. This gives rise to the phenomenon of double refraction. Because of this symmetry lowering, transparent crystals calcite are birefringent, as illustrated below.Ĭalcite crystals are birefringent, meaning that their refractive indices are different along the two principal crystal directions.

The fourfold rotation symmetry of the NaCl unit cell is lost when the spherical Cl - ions are replaced by triangular CO 3 2 - ions. From this image we can see why the CaCO 3 structure has a lower symmetry than that of NaCl. As in NaCl, each ion is coordinated by six of the other kind.
Triangular CO 3 2 - ions fill octahedral holes between the Ca 2 + ions (black spheres) in a distorted NaCl lattice. The calcite (CaCO 3) crystal structure is shown above. The rhombohedral unit cell of the calcite crystal structure. CaCO 3 (calcite, limestone, marble): Ca 2 + and triangular CO 3 2.CaC 2 (a salt-like carbide): Ca 2 + and linear C 2 2 - anions.FeS 2 (pyrite, "fools gold"): S 2 2 - (disulfide) and Fe 2 +.There are a number of compounds that have structures similar to that of NaCl, but have a lower symmetry (usually imposed by the geometry of the anion) than NaCl itself.


 0 kommentar(er)
0 kommentar(er)
